Regarding the current COVID-19 pandemic
almost 4 years ago
almost 4 years ago
Institute for Medical Informatics, Statistics and Epidemiology (IMISE) Current Bulletin on the COVID-19 pandemic in Leipzig and Saxony, 28 Nov. 2022 (in German).
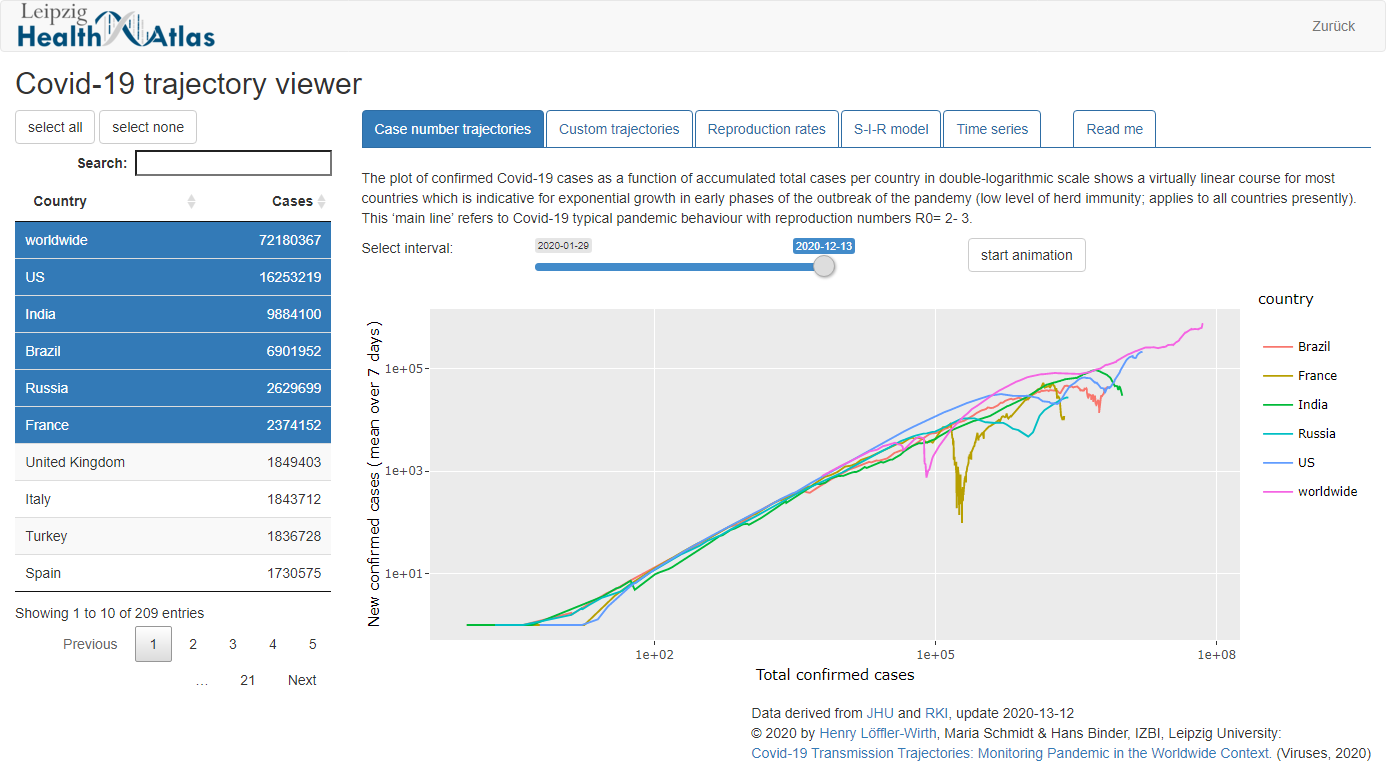
Additionally, the App "Covid-19 Viewer" is available, which provides an intuitive and interactive tool to monitor the development of the pandemic in 206 countries using simple plots.
The Health Atlas is now a product of NFDI4Health, the National Research Data Infrastructure for Personal Health Data. As "Local Data Hub/Leipzig", all content of the Health Atlas remains sustainable.
Gradually, you will be given further opportunities to enter and publish content within the framework of the.NFDI4Health or install an Local Data Hub in your institution - for more information, see:
LDH (in German)
Latest additions
 Spatial transcriptomics of human sebaceous gland hyperplasia
Spatial transcriptomics of human sebaceous gland hyperplasia
Data file - added 3 months ago Supplementary Information: Extended Table 3
Supplementary Information: Extended Table 3
Data file - added 4 months ago Supplementary Information: Extended Table 1
Supplementary Information: Extended Table 1
Data file - added 4 months ago Summary Statstics for vaspin ALL
Summary Statstics for vaspin ALL
Data file - added 5 months ago Summary Statistics
Summary Statistics
Resource - added 5 months ago GWAMA vaspin
GWAMA vaspin
Study - added 5 months ago SCALE-TORT Database
SCALE-TORT Database
Data file - added 7 months ago Prediction of Intensive Care Unit Length of Stay in the MIMIC-IV Dataset
Prediction of Intensive Care Unit Length of Stay in the MIMIC-IV Dataset
Publication - added 7 months ago
Find content
By tag: [show all]
1p and 19q co-deletion 3lgm2 3LGM² Tool acceptable change limit acquisition of phenotype data actigraph Actigraphy acylcarnitines addiction adenocarcinoma (in situ) adolescents adults advance care planning advance directives affect age age related macular degeneration ageing Agent-based modelling aggressive lymphoma aggressive non-hodgkin's lymphoma aging alzheimer's disease ambulance management amino acids amyloid anaemia ankle-brachial index anthracyclines anthropometric data anthropometry antibiotic treatment antidepressant medication anxiety apache uima apc arachidonic acid arousal array-based comparative genomic hybri... arterial stiffness associative memory asthma astrocytoma atrophy auditory evoked potentials automatic data processing b-cell lymphoma b-cell maturation bdnf behavioral variant frontotemporal dem... bethesda guidelines bioconductor bioinformatics biomarker biomathematical model Biomedical Ontologies biomedical ontology biometry bipolar blood disturbances blood transcriptome blood-oxygenation-level dependent body height body mass index boolean implications brain brain arousal regulation brain connectivity brain tumor brain-derived neurotrophic factor brainstem brca brca1 brca2 breast cancer cancer cancer genetics cancer heterogeneity cancer risk cancer syndromes cardiorespiratory fitness cardiotoxicity carotid artery plaque causal inference cdisc odm chemotherapy childhood obesity children choep cholinergic system chop chronotypes circadian rhythm cis-eQTL classification classifying cancer clinical data clinical diagnostic criteria clinical genetics clinical research informatics clinical trials co-regulated genes cognition cognitive abilities cognitive complaints cognitive control cognitive decline cognitive functioning cognitive functions cognitive neuropsychiatry cognitive reserve cognitive restrain cohort studies colloids colon cancer colorectal cancer colorectal neoplasm community-acquired pneumonia severity comt val158met Conditional Power Analysis confounding connectivity consciousness consense clustering Consent Management construct validity coronary artery disease correlated gene sets coupling between energy metabolism an... covid-19 cryptococcus neoformans crystalloids ctakes curating scientific data darbepoetin Data analysis data errors data integration Data Management data sharing data visualization daytime sleepiness deep-brain stimulation demands dementia depression development dhea-s diabetes mellitus diabetic retinopathy diagnostic criteria differentiation diffuse large B-cell lymphoma disinhibition dlbcl dna methylation dna methylation biomarkers Domain-specific Language dorsolateral prefrontal cortex dose-dense rituximab double-hit lymphoma drd4 exon iii vntr dried whole blood drug allergy early detection early intervention eating behaviour eating disorders ebv ecg edta-plasma education eeg eeg synchrony eicosanoids eigenvector centrality elderly electronic health records eligibility determination eloreta emotion emotional eating environmental data epidemiology epigenetic regulation epigenetic reprogramming epigenetics epo epworth sleepiness scale eQTL errors eSNP estradiol estrogens evaluation event-related fmri excel executive functions exercise testing expression quantitative trait locus Eye Disease faces fair FAIR Data fair4health false discovery rate family-wise error fdg fdg-pet fertility fhir filgrastim fmri follicular lymphoma format errors frequency frontotemporal lobar degeneration functional connectivity functional enrichment functional magnetic resonance imaging fungal allergy g-csf gene expression gene expression analysis gene expression data gene expression profiles gene regulation gene regulatory networks gene signatures gene targeting general population genetic counselling genetic screening Genetical Statistics genetics genomic imbalances genomic range geriatrics germinal center GFO glaucoma glia glioblastoma glioma global workspace globus pallidus glycosylated hemoglobin gonadotoxicity grade 3 grading gray matter density gray matter volume grey matter atrophy gwas H3K27me3 H3K4me3 hand grip strength hashimoto's thyroiditis hboc head and neck squamous cell carcinoma head motion health information interoperability Health Level Seven healthy adults heart failure hepatic macrophages hereditary hereditary cancer high dimensional data portraying hippocampal subfields hippocampus HL7 hnpcc hormonal transition periods Html human liver human papillomavirus human study hydrocortisone hyperarousal hypothyroidism idh1 idh1 mutation igh IHE illumina ht-12 v4 expression beadchips image processing images imaging puls sequence immune editing immune suppression immunohistochemistry in vitro liver model incidence incident dementia independent component analysis infections inflammation inflammatory cells inflammatory liver diseases information retrieval information storage Information Storage and Retrieval information storage and retrival insomnia insulin integration approach integrative bioinformatics integrative scientific data management intensity dependence inter-assay precision inter-institutional workflows Interoperability intestinal stem cell specification intima-media thickness intracranial ependymoma isocitrate dehydrogenase java knowledge bases Kupffer cells language large B-cell lymphoma late life late life depression leucopenia leukocyte subsets life life style lifestyle data Linux lipolysis liquid biopsy liver cell isolation logopenic progressive aphasia long-term survival longitudinal cohort study loudness dependence lps lymphoma lynch syndrome machine learning macrophage heterogeneity macrophages magnetic resonance imaging magnetization-prepared rapid gradient... major depression maldi malignant lymphomas manchester scoring system mania Mathematica mathematical model Mathematical Statistics maximum oxygen uptake mci medial prefrontal cortex medical informatics Medical Informatics Initiative medical sciences melanoma memory complaints mendelian randomisation Mendelian Randomization menopause menstrual cycle mental demands mental health meropenem meta-analysis metabolites metabolomics metadata metadata management metadata repository Method Development methylation of histone-lysine side ch... methylcap-seq mgmt microlesion effect mild cognitive impairment minor depression mismatch repair mismatch-repair defects mitosis model Modeling Information System Architecture Modeling Information System Architecture models module molecular function molecular oncology molecular profiling molecular subtypes monocyte-derived macrophages monomorphic montreal neurological institute mood mood disorder morbidity morphology mortality motivation motor cortex motor part of unified parkinson's dis... motor skills moxifloxacin mQTL mri multicolor flow cytometry multivariate analysis mutation prevalences myc n1-p2 n100 ncc neck metastasis network analysis neurobehavioral disorder neuroimaging neuron-specific enolase neurons neuropsychiatric disorders neuropsychiatry neuropsychology neurotransmitters nf-kb nicotinamide nlp non-coding RNA non-hodgkin lymphoma noncoronary atherosclerosis nonlinear lagged coherence normative data normative values novelty nse obesity occupation ocular biometry ocular fundus photography old people old-age oldest-old age oligodendroglioma ontology Ontology Matching ophthalmology optical coherence tomography organogenesis outcomes ovarian cancer ovarian failure overall survival p200 p53 parkinson's disease pathway activity pathway enrichment pathway signal flow pattern classification pbmc pcsk9 pct peak oxygen uptake pediatric pedunculopontine nucleus pegfilgrastim perception peripheral blood peripheral blood monocyte perl pet pharmacotherapy Phenotype phenotype calculation phenotype classification phenotype definition phenotype ontology phenotype reasoning phenotyping phq-15 physical activity phytosterols pipeline plasticity plasticity of cell function polygenetic regulation polymorphic polypharmacy population-based study power of attorney PPRL preclinical alzheimer's disease preprocessing prevalence prevention primary care primary human liver cells primary progressive aphasia progesterone prognosis prognostic factors prognostic impact progression risk progression-free survival progressive nonfluent aphasia promoter methylation pseudotime Psychiatry psychological factors puls wave velocity pwv pwv data Python quantitative methylation-specific pcr questionnaire r r-chop radiofrequence coil Record Linkage recruitment reference data reference intervals refraction regulation of gene expression relapse repeated measures analysis of variance Requirements Engineering response resting-state fmri restingstate restrained eating retinal imaging retinal imaging retinal nerve fiber layer Retinal Nerve Fiber Layer Thickness ricover-60 risk criteria risk factors risk prediction models rituximab rituximab dose rituximab pharmacokinetics rituximab toxicity RNFLT rs-fmri rs4680 ruby s100b Schema Matching schizophrenia screening secular trends Selection Criteria self organizing maps self-organizing map self-regulation semantic dementia semantic errors septic shock sequencing seroreactivity serum serum marker severe sepsis sex sexual dimorphism shiny Single Cell modelling single-cell transcriptomics sleep sleep disturbance sleep quality small-vessel disease smoking snp snp data social isolation social network socio-economic status sodium selenite som som data somatic mutations somatic symptoms spatial visualization specimen acquisition and tracking spss stability standard deviation Statistical Consulting statistics stem cells stem-cell differentiation stemness step test steroid hormones study items subgenual prefrontal cortex subjective cognition subjective cognitive decline subjective cognitive impairment subjective memory complaints submission subthalamic nucleus Summary Statistics support vector machine survey survival syntax errors synthea Teaching testosterone text mining Theoretical Physics three-factor eating questionnaire thyroidperoxidase antibodies time trends tissue engineering tissue-specific top-level ontology tp53 tp53 mutation trans-cluster trans-eQTL transcription transcriptome and methylome transcriptomics translocation tumor progression tumor suppressor gene tumour heterogeneity ubiquitin uncontrolled eating user acceptance usp9x uterine cervical neoplasms validation vascular cognitive impairment vbm vigall vigall 2.1 vigilance Vigilance Regulation vision science visual acuity visual impairments visualization vo2max vo2peak voice range profile voxel size voxel-based morphometry vsaq questionnaire Web Archive web portal white matter disease white matter hyperintensities words work work environment work packages and tools workflow writers and erasers of epigenetic marks xiap ymca-step test
Tags
[show all]
1p and 19q co-deletion 3lgm2 3LGM² Tool acceptable change limit acquisition of phenotype data actigraph Actigraphy acylcarnitines addiction adenocarcinoma (in situ) adolescents adults advance care planning advance directives affect age age related macular degeneration ageing Agent-based modelling aggressive lymphoma aggressive non-hodgkin's lymphoma aging alzheimer's disease ambulance management amino acids amyloid anaemia ankle-brachial index anthracyclines anthropometric data anthropometry antibiotic treatment antidepressant medication anxiety apache uima apc arachidonic acid arousal array-based comparative genomic hybri... arterial stiffness associative memory asthma astrocytoma atrophy auditory evoked potentials automatic data processing b-cell lymphoma b-cell maturation bdnf behavioral variant frontotemporal dem... bethesda guidelines bioconductor bioinformatics biomarker biomathematical model Biomedical Ontologies biomedical ontology biometry bipolar blood disturbances blood transcriptome blood-oxygenation-level dependent body height body mass index boolean implications brain brain arousal regulation brain connectivity brain tumor brain-derived neurotrophic factor brainstem brca brca1 brca2 breast cancer cancer cancer genetics cancer heterogeneity cancer risk cancer syndromes cardiorespiratory fitness cardiotoxicity carotid artery plaque causal inference cdisc odm chemotherapy childhood obesity children choep cholinergic system chop chronotypes circadian rhythm cis-eQTL classification classifying cancer clinical data clinical diagnostic criteria clinical genetics clinical research informatics clinical trials co-regulated genes cognition cognitive abilities cognitive complaints cognitive control cognitive decline cognitive functioning cognitive functions cognitive neuropsychiatry cognitive reserve cognitive restrain cohort studies colloids colon cancer colorectal cancer colorectal neoplasm community-acquired pneumonia severity Computer Science comt val158met Conditional Power Analysis confounding connectivity consciousness consense clustering Consent Management construct validity coronary artery disease correlated gene sets coupling between energy metabolism an... covid-19 cryptococcus neoformans crystalloids ctakes curating scientific data darbepoetin Data analysis data errors data integration Data Management data sharing data visualization daytime sleepiness deep-brain stimulation demands dementia depression development dhea-s diabetes mellitus diabetic retinopathy diagnostic criteria differentiation diffuse large B-cell lymphoma disinhibition dlbcl dna methylation dna methylation biomarkers Domain-specific Language dorsolateral prefrontal cortex dose-dense rituximab double-hit lymphoma drd4 exon iii vntr dried whole blood drug allergy early detection early intervention eating behaviour eating disorders ebv ecg edta-plasma education eeg eeg synchrony eicosanoids eigenvector centrality elderly electronic health records eligibility determination eloreta emotion emotional eating environmental data epidemiology epigenetic regulation epigenetic reprogramming epigenetics epo epworth sleepiness scale eQTL errors eSNP estradiol estrogens evaluation event-related fmri excel executive functions exercise testing expression quantitative trait locus Eye Disease faces fair FAIR Data fair4health false discovery rate family-wise error fdg fdg-pet fertility fhir filgrastim fmri follicular lymphoma format errors frequency frontotemporal lobar degeneration functional connectivity functional enrichment functional magnetic resonance imaging fungal allergy g-csf gene expression gene expression analysis gene expression data gene expression profiles gene regulation gene regulatory networks gene signatures gene targeting general population genetic counselling genetic screening Genetical Statistics genetics genomic imbalances genomic range geriatrics germinal center GFO glaucoma glia glioblastoma glioma global workspace globus pallidus glycosylated hemoglobin gonadotoxicity grade 3 grading gray matter density gray matter volume grey matter atrophy gwas H3K27me3 H3K4me3 hand grip strength hashimoto's thyroiditis hboc head and neck squamous cell carcinoma head motion health information interoperability Health Level Seven healthy adults heart failure hepatic macrophages hereditary hereditary cancer high dimensional data portraying hippocampal subfields hippocampus HL7 hnpcc hormonal transition periods Html human liver human papillomavirus human study hydrocortisone hyperarousal hypothyroidism idh1 idh1 mutation igh IHE illumina ht-12 v4 expression beadchips image processing images imaging puls sequence immune editing immune suppression immunohistochemistry in vitro liver model incidence incident dementia independent component analysis infections inflammation inflammatory cells inflammatory liver diseases information retrieval information storage Information Storage and Retrieval information storage and retrival insomnia insulin integration approach integrative bioinformatics integrative scientific data management intensity dependence inter-assay precision inter-institutional workflows Interoperability intestinal stem cell specification intima-media thickness intracranial ependymoma isocitrate dehydrogenase java KDS kerndatensatzkonform knowledge bases Kupffer cells language large B-cell lymphoma late life late life depression leucopenia leukocyte subsets life life style lifestyle data Linux lipolysis liquid biopsy liver cell isolation logopenic progressive aphasia long-term survival longitudinal cohort study loudness dependence lps lymphoma lynch syndrome machine learning macrophage heterogeneity macrophages magnetic resonance imaging magnetization-prepared rapid gradient... major depression maldi malignant lymphomas manchester scoring system mania Mathematica mathematical model Mathematical Statistics maximum oxygen uptake mci medial prefrontal cortex medical informatics Medical Informatics Initiative medical sciences melanoma memory complaints mendelian randomisation Mendelian Randomization menopause menstrual cycle mental demands mental health meropenem meta-analysis metabolites metabolomics metadata metadata management metadata repository Method Development methylation of histone-lysine side ch... methylcap-seq mgmt microlesion effect mild cognitive impairment minor depression mismatch repair mismatch-repair defects mitosis model Modeling Information System Architecture Modeling Information System Architecture models module molecular function molecular oncology molecular profiling molecular subtypes monocyte-derived macrophages monomorphic montreal neurological institute mood mood disorder morbidity morphology mortality motivation motor cortex motor part of unified parkinson's dis... motor skills moxifloxacin mQTL mri multicolor flow cytometry multivariate analysis mutation prevalences myc n1-p2 n100 ncc neck metastasis network analysis neurobehavioral disorder neuroimaging neuron-specific enolase neurons neuropsychiatric disorders neuropsychiatry neuropsychology neurotransmitters nf-kb nicotinamide nlp non-coding RNA non-hodgkin lymphoma noncoronary atherosclerosis nonlinear lagged coherence normative data normative values novelty nse obesity occupation ocular biometry ocular fundus photography old people old-age oldest-old age oligodendroglioma ontology Ontology Matching ophthalmology optical coherence tomography organogenesis outcomes ovarian cancer ovarian failure overall survival p200 p53 parkinson's disease pathway activity pathway enrichment pathway signal flow pattern classification pbmc pcsk9 pct peak oxygen uptake pediatric pedunculopontine nucleus pegfilgrastim perception peripheral blood peripheral blood monocyte perl pet pharmacotherapy Phenotype phenotype calculation phenotype classification phenotype definition phenotype ontology phenotype reasoning phenotyping phq-15 physical activity phytosterols pipeline plasticity plasticity of cell function polygenetic regulation polymorphic polypharmacy population-based study power of attorney PPRL preclinical alzheimer's disease preprocessing prevalence prevention primary care primary human liver cells primary progressive aphasia progesterone prognosis prognostic factors prognostic impact progression risk progression-free survival progressive nonfluent aphasia promoter methylation pseudotime Psychiatry psychological factors puls wave velocity pwv pwv data Python quantitative methylation-specific pcr questionnaire r r-chop radiofrequence coil Record Linkage recruitment reference data reference intervals refraction regulation of gene expression relapse repeated measures analysis of variance Requirements Engineering response resting-state fmri restingstate restrained eating retinal imaging retinal imaging retinal nerve fiber layer Retinal Nerve Fiber Layer Thickness ricover-60 risk criteria risk factors risk prediction models rituximab rituximab dose rituximab pharmacokinetics rituximab toxicity RNFLT rs-fmri rs4680 ruby s100b Schema Matching schizophrenia screening secular trends Selection Criteria self organizing maps self-organizing map self-regulation semantic dementia semantic errors septic shock sequencing seroreactivity serum serum marker severe sepsis sex sexual dimorphism shiny Single Cell modelling single-cell transcriptomics sleep sleep disturbance sleep quality small-vessel disease smoking snp snp data social isolation social network socio-economic status sodium selenite som som data somatic mutations somatic symptoms spaCy spatial visualization specimen acquisition and tracking spss stability standard deviation Statistical Consulting statistics stem cells stem-cell differentiation stemness step test steroid hormones study items subgenual prefrontal cortex subjective cognition subjective cognitive decline subjective cognitive impairment subjective memory complaints submission subthalamic nucleus Summary Statistics support vector machine survey survival syntax errors synthea Teaching Test Data Testdaten testosterone text mining Theoretical Physics three-factor eating questionnaire thyroidperoxidase antibodies time trends tissue engineering tissue-specific top-level ontology tp53 tp53 mutation trans-cluster trans-eQTL transcription transcriptome and methylome transcriptomics translocation tumor progression tumor suppressor gene tumour heterogeneity ubiquitin uncontrolled eating user acceptance usp9x uterine cervical neoplasms validation vascular cognitive impairment vbm vigall vigall 2.1 vigilance Vigilance Regulation vision science visual acuity visual impairments visualization vo2max vo2peak voice range profile voxel size voxel-based morphometry vsaq questionnaire Web Archive web portal white matter disease white matter hyperintensities words work work environment work packages and tools workflow writers and erasers of epigenetic marks xiap ymca-step test
Content tagged with Human Disease concepts
Untagged:
Projects: 27, Publications: 628, Models: 16, Data files: 347
Projects: 27, Publications: 628, Models: 16, Data files: 347
